- How Is Atomic Number Found
- Atomic Number Of An Atom Definition
- Cached
- Atomic Number Of An Atom Vs Mass Number
The atomic number of an atom represents the number of protons found in the nucleus of that atom. Elements are identified by their atomic numbers because each element has a different number of protons in its nucleus. The periodic table of the elements is organized by atomic numbers.
Atomic Structure SPS1. Students will investigate our current understanding of the atom. Examine the structure of the atom in terms of – proton, electron, and neutron locations. – atomic mass and atomic number. – atoms with different numbers of neutrons (isotopes). – explain the relationship of the proton number to the element’s identity. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons. Fundamental properties of atoms including atomic number and atomic. Ans: Atomic number = 3 Explanation:The illustrated atom is of group 1 of Alkali metal in the periodi view the full answer.
The atomic number (symbol: Z) of an atom is the number of protons in the nucleus of the atom.[1][2] The atomic number of an atom identifies which element it is. In a neutral atom, the atomic number is equal to the number of electrons orbiting the nucleus.[1] The elements of the periodic table are listed in order of increasing atomic number.[2]
:max_bytes(150000):strip_icc()/atomic-mass-and-mass-number-606105_v1-80df956ab98440bc9969531d1bb6c874.png)
Atomic number is not the same as:
- atomic mass (symbol: ma), which is the mass of a single atom, commonly expressed in unified atomic mass units
- mass number (symbol: A), which is the sum of the number of protons and number of neutrons in the nucleus of an atom
- relative atomic mass (also called atomic weight;symbol: Ar), which is the ratio of the average mass per atom of an element from a given sample to 1/12 the mass of a carbon-12 atom.
The atomic number of the periodic table directly corresponds to the number of protons which is in the atom. Once another proton is added, it is no longer the same element. The same cannot be applied to when another neutron or another electron is added. Adding more electrons will give the atom a negative charge and removing electrons will give the atom a positive charge. Metals tend to lose electrons, which creates a positive charge. Non-metals tend to gain electrons, forming a negative charge. Electrons are the foundation for determining how compounds are formed amongst atoms. Adding or removing neutrons within an atom changes its isotope. As an example, carbon-12 is the most stable isotope for a carbon atom. However, we can add two more neutrons and carbon-12 is now carbon-14, a less stable isotope of carbon. The number of an isotope directly correlates to the atomic mass of an element. The amount of neutrons in any given atom by subtracting the atomic number from the atomic mass.
How Is Atomic Number Found

References[change | change source]
- ↑ 1.01.1Daintith, John, ed. (2008). A Dictionary of Chemistry (Sixth ed.). Oxford University Press. p. 48. ISBN978-0-19-920463-2.
- ↑ 2.02.1Moore, John T. (2010). Chemistry Essentials For Dummies. Wiley. p. 36. ISBN978-0-470-61836-3.
Related pages[change | change source]
Learning Outcomes
- Define atomic and mass numbers.
- Determine the number of protons, neutrons, and electrons in an atom.
- Identify the charge and relative mass of subatomic particles.
- Label the location of subatomic particles in the atom.
- Determine the mass of an atom based on its subatomic particles.
- Write A/Z and symbol-mass format for an atom.
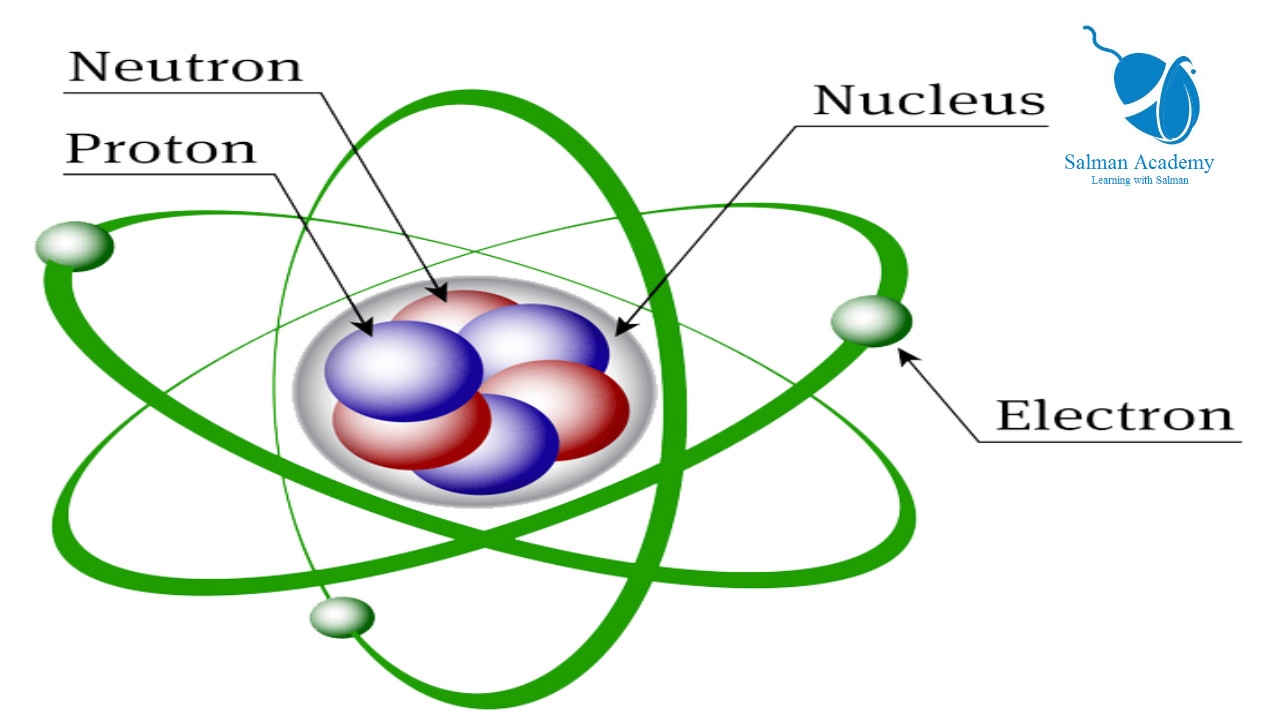
Atoms are the fundamental building blocks of all matter and are composed of protons, neutrons, and electrons. Because atoms are electrically neutral, the number of positively charged protons must be equal to the number of negatively charged electrons. Since neutrons do not affect the charge, the number of neutrons is not dependent on the number of protons and will vary even among atoms of the same element.
Atomic Number

The atomic number (represented by the letter Z)of an element is the number of protons in the nucleus of each atom of that element. An atom can be classified as a particular element based solely on its atomic number. For example, any atom with an atomic number of 8 (its nucleus contains 8 protons) is an oxygen atom, and any atom with a different number of protons would be a different element. The periodic table (see figure below) displays all of the known elements and is arranged in order of increasing atomic number. In this table, an element's atomic number is indicated above the elemental symbol. Hydrogen, at the upper left of the table, has an atomic number of 1. Every hydrogen atom has one proton in its nucleus. Next on the table is helium, whose atoms have two protons in the nucleus. Lithium atoms have three protons, beryllium atoms have four, and so on.
Since atoms are neutral, the number of electrons in an atom is equal to the number of protons. Hydrogen atoms all have one electron occupying the space outside of the nucleus. Helium, with two protons, will have two electrons. In the chemical classroom, the proton count will always be equivalent to an atom's atomic number. This value will not change unless the nucleus decays or is bombarded (nuclear physics).
Mass Number
Experimental data showed that the vast majority of the mass of an atom is concentrated in its nucleus, which is composed of protons and neutrons. The mass number (represented by the letter A)is defined as the total number of protons and neutrons in an atom. Consider the table below, which shows data from the first six elements of the periodic table.
| Name | Symbol | Atomic Number (Z) | Protons | Neutrons | Electrons | Mass Number (A) (rounded to two decimals) |
|---|---|---|---|---|---|---|
| hydrogen | (ce{H}) | 1 | 1 | 0 | 1 | 1.01 |
| helium | (ce{He}) | 2 | 2 | 2 | 2 | 4.00 |
| lithium | (ce{Li}) | 3 | 3 | 4 | 3 | 6.94 |
| beryllium | (ce{Be}) | 4 | 4 | 5 | 4 | 9.01 |
| boron | (ce{B}) | 5 | 5 | 6 | 5 | 10.18 |
| carbon | (ce{C}) | 6 | 6 | 6 | 6 | 12.01 |
Consider the element helium. Its atomic number is 2, so it has two protons in its nucleus. Its nucleus also contains two neutrons. Since (2 + 2 = 4), we know that the mass number of the helium atom is 4. Finally, the helium atom also contains two electrons, since the number of electrons must equal the number of protons. This example may lead you to believe that atoms have the same number of protons and neutrons, but a further examination of the table above will show that this is not the case. Lithium, for example, has three protons and four neutrons, giving it a mass number of 7.
Knowing the mass number and the atomic number of an atom allows you to determine the number of neutrons present in that atom by subtraction.
Atomic Number Of An Atom Definition
[text{Number of neutrons} = text{ rounded mass number} - text{atomic number}]
Atoms of the element chromium (left( ce{Cr} right)) have an atomic number of 24 and a mass number of 52. How many neutrons are in the nucleus of a chromium atom? To determine this, you would subtract as shown:
[52 - 24 = 28 : text{neutrons in a chromium atom}]
The composition of any atom can be illustrated with a shorthand notation called A/Z format. Both the atomic number and mass are written to the left of the chemical symbol. The 'A' value is written as a superscript while the 'Z' value is written as a subscript. For an example of this notation, look to the chromium atom shown below:
[ce{^{52}_{24}Cr}]
Another way to refer to a specific atom is to write the mass number of the atom after the name, separated by a hyphen. Symbol-mass format for the above atom would be written as Cr-52. In this notation, the atomic number is not included. You will need to refer to a periodic table for proton values.
Example (PageIndex{1})
Calculate each of the three subatomic particles and give specific group or period names for each atom.
Cached
- mercury
- platinum
- bromine
Solutions
- Hg (transition metal)- has 80 electrons, 80 protons, and 121 neutrons
- Pt (transition metal)- has 78 electrons, 78 protons, and 117 neutrons
- Br (halogen)- has 35 electrons, 35 protons, and 45 neutrons
Example (PageIndex{2})
Write both A/Z and symbol-mass formats for the atoms in Example (PageIndex{1}).
Solutions
- (ce{^{201}_{80}Hg}) and Hg-201
- (ce{^{195}_{78}Pt}) and Pt-195
- (ce{^{80}_{35}Br}) and Br-80
Example (PageIndex{3})
Identify the elements based on the statements below.
- Which element has 25 protons?
- Which element has 0 neutrons?
- Which element has 83 electrons?
Solutions
a. manganese
b. hydrogen
c. bismuth
Need More Practice?
- Turn to section 3.E of this OER and answer questions #1-#2, #4, and #8.
Contributors and Attributions
Atomic Number Of An Atom Vs Mass Number
CK-12 Foundation by Sharon Bewick, Richard Parsons, Therese Forsythe, Shonna Robinson, and Jean Dupon.
Allison Soult, Ph.D. (Department of Chemistry, University of Kentucky)
